Stakeholder
Clinical trials data publication now more transparent in the EU
For patients with a life-threatening disease, clinical trials can sometimes offer a lifeline by allowing enrolment in new treatments.
“And if you're a patient with a fatal condition, time lag when finding out about a potentially life-saving clinical trial is not something you want,” Peter Arlett, head of data analytics and methods task force at the European Medicines Agency (EMA), says.
A new version of the portal called the Clinical Trial Information System (CTIS) aims to offer relief under revised transparency rules that became applicable on 18 June 2024.
CTIS is a portal established under the Clinical Trials Regulation (CTR), which came into force in January 2022. The regulation on the one hand creates a clear legal framework for each member state that covers a new approval and assessment process for clinical trials, and on the other hand, allows for more transparency and input for clinical trials.
The CTIS portal, which is maintained by EMA, brings together two main functions. First, it’s the single entry point for submission and assessment of clinical trials in the EU, both for applicants (so-called ‘sponsors’) and regulators. This means that companies or academics developing new treatments no longer have to apply for trial authorisation in each member state, considerably reducing bureaucratic overhead, and using clear standards for required documentation and transparency.
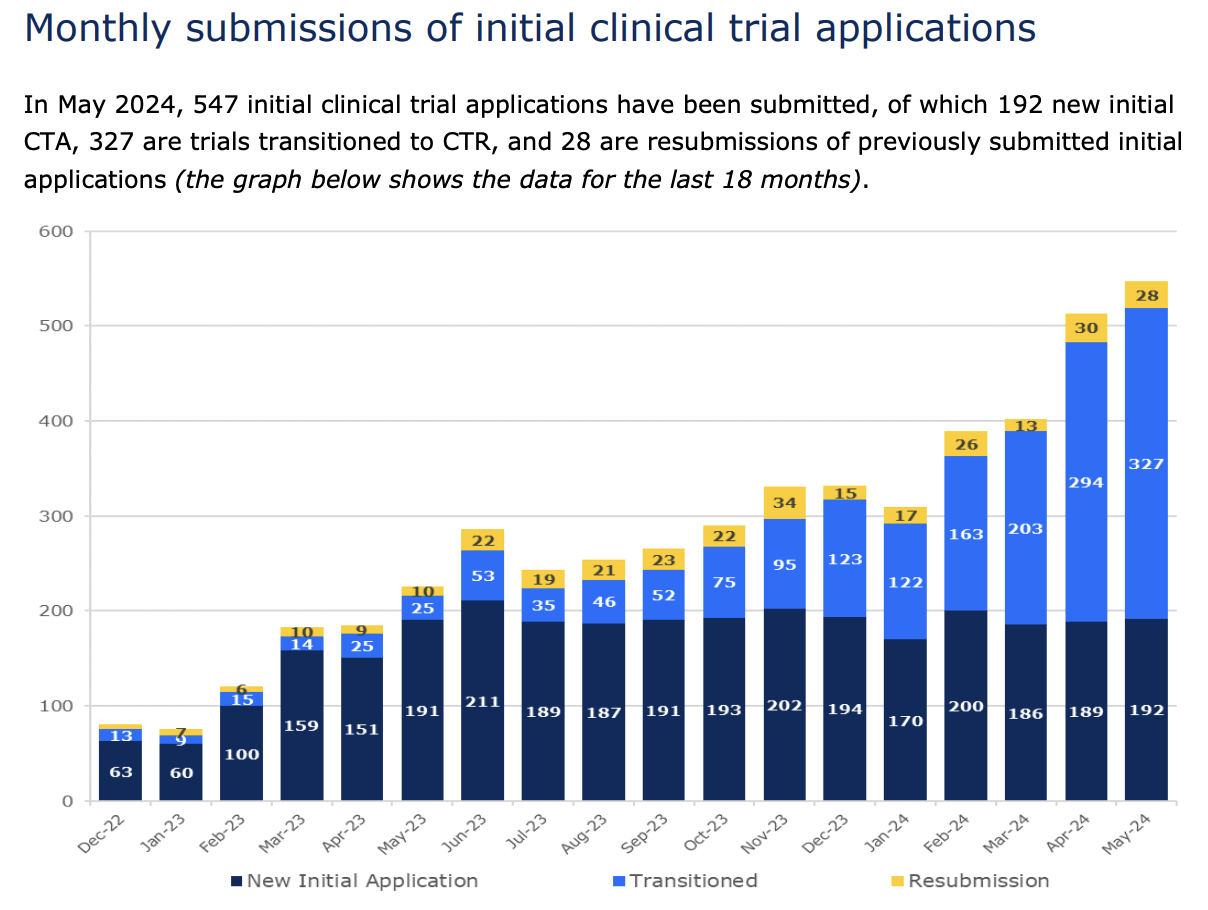
Second, CTIS offers a public and searchable database of clinical trials for healthcare professionals, patients and the general public. Since all clinical trials that are to be conducted in the EU and European Economic Area (EEA) are now to be included on CTIS, healthcare professionals and patients alike can use this portal to search and find details on for example start and end date, the condition being treated, the country the trial is run in and the trial status.
According to the latest report on EU/EEA clinical trials, 5,508 clinical trials have been submitted through CTIS, 2,185 of which are ongoing. As of today, more than 4,400 trials have received a decision and are therefore publicly available. By 30 January 2025, all clinical trials in the EU must be moved to CTIS. This deadline comes three years after the regulation became applicable.
Arlett expects approximately 500 additional trials to be published every subsequent month. “We're seeing about 500 clinical trial approvals per month for the Clinical Trials Regulation. So by the end of July, we'll have another 500, by the end of August, another 500... And when they're approved, for the vast majority of them, there will be public granular information, including the trial protocol,” Arlett says.
Transparency rules
CTIS includes and makes public clinical trial application documents (e.g. protocol) as well as information such as the contact information of the trial sponsor, which is now mandatory to be publicly available.
Moreover, while under the former CTIS transparency rules clinical trial application documents could be subject to a deferral mechanism that would allow sponsors to delay their publishing for up to seven years to protect commercially confidential information, now, thanks to the revised transparency rules, those documents are published earlier in time, after authorisation and before even the start of the trial for most of the trials.
The former possibility to defer the publication of application documents, in the words of Arlett, was “clearly meeting the legal requirements to protect commercially confidential information,” but was “not following the spirit of transparency.”
“Today, the day after the authorisation of the clinical trial, the information on its protocol, together with relevant key documents of interest goes into the public domain,” Arlett says.
The updated transparency rules are intended to strike a balance between the protection of personal and commercially sensitive information from sponsors – who can redact information deemed sensitive – and transparency of information by releasing important clinical trial information earlier.
Great care has been taken on the public-facing side of CTIS to make it user-friendly for healthcare professionals and patients alike to find the information they need and increase awareness of upcoming or promising treatment options.
“We are in an age of data and knowledge, aren't we? And the centralised system of submitting, assessing, authorising, and making public information about clinical trials in the EU will be a very rich source, not just for us as regulators, but also for companies, academics, and patients associations,” he adds.
The stakeholders Arlett names were all consulted in the development of CTIS, says María Jesús Lamas, who is director of the Spanish Agency of Medicines and Health Products (AEMPS), and regulatory co-chair of the ACT EU multi-stakeholder platform advisory group (MSP AG).
ACT EU stands for Accelerating Clinical Trials in the EU, and is a collaboration between the European Medicines Agency (EMA), the Heads of Medicines Agencies (HMA) in the member states and the European Commission, seeking to transform how clinical trials are initiated, designed, and run.
The initiative supports and brings together academic sponsors, regulators, patients and healthcare professionals in creating processes to improve how clinical trials are done in the EU.
“Indeed, we bring together all the stakeholders that could be involved somehow in clinical trials. So, on the regulatory side, the assessors of trials and then on the other side academic sponsors of clinical trials, industry, patients, healthcare professionals, clinical pharmacists, physicians, representatives of ethics, and ethics committees. So, I would say that everybody that has a word in conducting clinical trials is involved,” Lamas says.
Lamas agrees that it was not always easy for patients to find clinical trials taking place in other member states before CTIS. In Spain, it’s possible for patients to search for specific clinical trials, by disease or by medicines involved. But those platforms did not cover trials authorised in other countries. “It has a clear practical benefit for patients, because they will be able to look for and find clinical trials for rare diseases or in situations in which there are no alternatives within the whole European Union,” she says.
Change requires work
Lamas does stress that the user-experience of the platform is being constantly improved, and that the stakeholder advisory group will play a crucial role in providing feedback for both the way the platform works and for its many different users.
From the side of multinational pharmaceutical companies, “the benefits are very clear,” Arlett says. Rather than submitting requests in multiple languages under multiple legislations, there’s a single process. “But change requires work,” Arlett also says, for example for individual researchers who are used to submitting their applications to their national authorities in their own language.
To help ease the burden, EMA has published not only extensive documentation on how to use the CTIS platform, but also a comprehensive library of support material, including videos, which cover every step of using CTIS throughout a clinical trial.
Moreover, the secure submission portal of CTIS is built with the idea to streamline and simplify the process of submission of new clinical trials for assessment.
Lamas adds here that although there is now a single portal for managing clinical trials, the assessment of individual clinical trials is still a national competence. “Assessors now have to rely on assessors in other member states, and have to accept that the assessment of trials is done by others,” she says. “It’s the first time we’re doing this with clinical trials, so sometimes assessments are not as streamlined or pragmatic as they should be. But it’s a process of mutual learning, building trust, and improving what can be done.”
Accelerating clinical trials
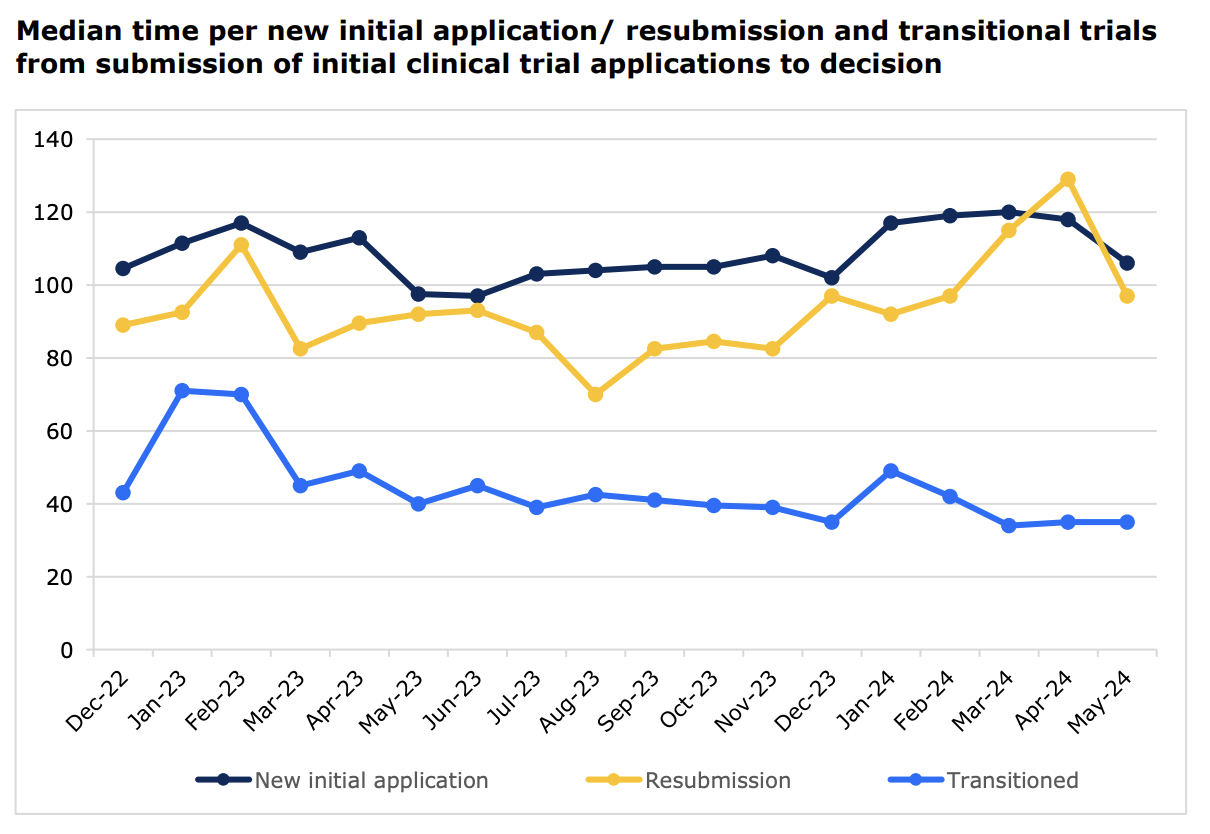
While some concerns remain, the latest report on key performance indicators of the CTIS platform have been encouraging. For instance, the median time in days between the initial submission of a clinical trial to a decision has, for commercial sponsors, dropped from around 110 days to just 50 days.
This improvement is even more remarkable considering that the number of monthly submissions has surged from just over 200 in May 2023 to over 500 in May 2024.
The hope and expectation is that CTIS will now encourage more and more transparent clinical trials research in the EU, but also provide a trove of data for medical researchers to find more information on new treatments currently being studied.
“We as regulators are working collectively with stakeholders, including patients, healthcare professionals and sponsors of both commercial and non-commercial clinical trials, to make the implementation of the clinical trials regulation a success. In addition, through ACT EU we want to invigorate the clinical trials environment in Europe so that more, and more impactful clinical trials are done in Europe,” Arlett concludes.
Disclaimer
This article is sponsored by a third party. All opinions in this article reflect the views of the author and not of EUobserver.Author Bio
The European Medicines Agency (EMA) is an agency of the European Union(EU) in charge of the evaluation and supervision of pharmaceutical products. This article was produced in collaboration with EUobserver.
Tags
Author Bio
The European Medicines Agency (EMA) is an agency of the European Union(EU) in charge of the evaluation and supervision of pharmaceutical products. This article was produced in collaboration with EUobserver.